In the field of medical device regulation, the NMPA Master Record Filing is a core regulatory system established by China's National Medical Products Administration (NMPA) for raw materials used in medical devices. This system requires raw material manufacturers to submit key technical documentation related to safety and efficacy to the regulatory authority. After official review and approval, a unique registration number is granted.
1. Pharnorcia UV090 CAS#96478-09-0
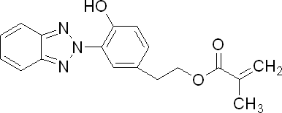
Product Name: 3-(2'-Hydroxy-5'-methacryloxyethylphenyl)-2H-benzotriazole
Molecular Formula: C18H17N3O3
Key Advantages:
Full-Spectrum Coverage: UV090 absorbs >99% of UVA (315–380 nm) and UVB (280–315 nm) radiation, covering the full 280–380 nm range. It extends the UV cutoff wavelength of lenses to380 nm, significantly outperforming traditional materials (ESPF index: 10–15) .
Selective Light Transmission: Its molecular structure ensures high selectivity for UV absorption while maintaining >99% visible light transmittance, preserving visual clarity .

Regulatory Significance:
Quality Endorsement: NMPA filing certifies official validation of production processes, quality control, and safety performance, providing downstream medical device manufacturers with authoritative proof of raw material reliability .
Technical Barrier: The filing system raises industry entry thresholds, ensuring critical medical materials meet unified high standards—especially vital for sensitive ocular-contact materials .
Pharnorcia UV090 (CAS#:96478-09-0)has completed this stringent review process, marking its status as a top-tier medical-grade raw material with proven quality reliability and regulatory compliance.
2. Pharnorcia: The "Invisible Champion" in Ophthalmic Materials
As a technology enterprise specializing in high-performance UV-blocking ophthalmic materials, Pharnorcia has accumulated 19 years of R&D and manufacturing expertise in optoelectronic materials, evolving into a trusted partner for global ophthalmic giants :
Technology Platform: The company has established a comprehensive ophthalmic materials platform, focusing on R&D and mass production of high-purity monomers and functional materials. Its portfolio spans high-end applications like silicone hydrogel contact lenses, intraocular lens (IOL) monomers, and core materials for orthokeratology (OK) lenses .
Global Recognition: Pharnorcia's technical capabilities have been rigorously validated by industry leaders, including Johnson & Johnson, Bausch + Lomb, CooperVision, and Alcon, which conducted nearly 20 on-site audits. It is a designated global supplier for multiple Fortune 500 companies .
Global Footprint: Products are exported to >50 developed countries, with thousands of batches of high-quality ophthalmic materials delivered, building a worldwide chain of trust .
 【Scan code】
【Scan code】

WeChat identification QR code