As the global ophthalmology industry rapidly evolves, the demand for high-performance UV-absorbing materials continues to grow. Pharnorcia® UV725 (CAS#:2035-72-5) stands out for its exceptional performance and reliable quality, earning the trust of many top-tier international ophthalmic companies.

Product Name: UV725
English Name: 4-Methacryloxy-2-hydroxybenzophenone
CAS#: 2035-72-5
Molecular Formula: C17H14O4
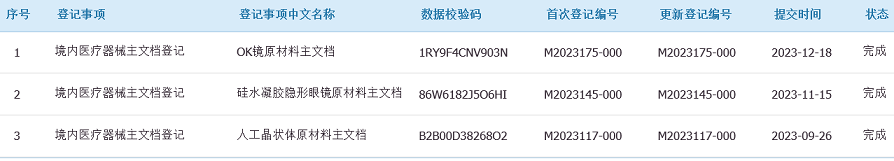
Pharnorcia® UV725 (CAS#:2035-72-5) is a material registered in the master file of the Chinese National Medical Products Administration (NMPA). It is widely used in orthokeratology (OK) lenses, intraocular lenses (IOLs), and silicone hydrogel contact lenses, making it a trusted, high-quality choice for ophthalmic applications.
Outstanding Product Advantages
1. Superior UV Absorption Performance
Pharnorcia® UV725 excels in UV absorption, effectively blocking UVA and UVB rays. This helps reduce potential UV damage to the eyes, especially for users wearing contact lenses for extended periods.
2. Stable Chemical Properties
The material demonstrates high stability across various processing environments. Whether exposed to high temperatures or stored for long durations, UV725 maintains its UV absorption performance.
3. Exceptional Biocompatibility
Validated through multiple biological tests, Pharnorcia® UV725 (CAS#:2035-72-5) is non-irritating to human ocular tissues, ensuring safety and comfort for end-users.
Global Market Presence
As a leading UV-absorbing material, Pharnorcia® UV725 (CAS#:2035-72-5) is exported to numerous countries and regions worldwide. It is widely applied in contact lenses and other ophthalmic products. Pharnorcia® UV725 has become a long-term supply material for globally renowned ophthalmic brands like Johnson & Johnson, Bausch + Lomb, and Alcon, showcasing its prominent position in the international market.
Efficient Global Supply Chain
Pharnorcia® has established an efficient supply network covering major markets in North America, Europe, and Asia, ensuring customers receive high-quality materials promptly.
Quality Assurance System
Pharnorcia® upholds a strict quality management philosophy. UV725 (CAS#:2035-72-5) has passed international quality management system certifications and is registered in the master file of the Chinese National Medical Products Administration (NMPA). This not only demonstrates the product's superior quality but also facilitates global customers in product registration and market entry.
Pharnorcia® UV725 (CAS#:2035-72-5) is a reliable choice for ophthalmic companies seeking high-performance UV-absorbing materials. With its exceptional UV absorption capabilities, superior quality, and global supply chain, it has become the trusted material for the world’s leading ophthalmic companies.
Contact Information:
Email: gm@osigen.com.cn
Phone: +86-13911254076
Website: www.pharnorcia.com
 【Scan code】
【Scan code】

WeChat identification QR code