As the global demand for high-performance ophthalmic materials continues to grow, Pharnorcia® VMA (CAS#:3195-78-6) has emerged as a trusted, high-quality material recognized worldwide for its superior performance and extensive international certifications.

Product Overview
Product Name: VMA
English Name: N-Vinyl-N-methylacetamide
CAS#: 3195-78-6
Molecular Formula: C5H9NO
Pharnorcia® VMA (CAS#:3195-78-6) is a material registered in the master file of the Chinese National Medical Products Administration (NMPA). It is widely used in orthokeratology (OK) lenses, intraocular lenses (IOLs), and silicone hydrogel contact lenses, making it a reliable and high-quality material for ophthalmic applications.
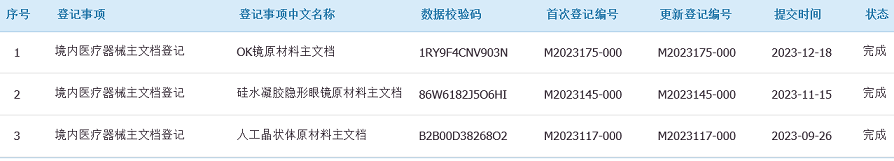
Advantages of Pharnorcia® VMA
1. Internationally Certified Quality
Pharnorcia® VMA (CAS#:3195-78-6) complies with international quality management system certifications, meeting the rigorous requirements of the global ophthalmology industry. It provides unmatched quality assurance for customers.
2. High Purity and Stability
Using advanced purification technology, VMA achieves exceptional purity and demonstrates outstanding chemical stability across various application scenarios, ensuring reliable performance.
3. Superior Biocompatibility
Extensive biocompatibility testing confirms that VMA (CAS#:3195-78-6) is gentle and non-irritating to ocular tissues. This makes it an ideal choice for developing premium contact lenses and optical products.
Global Market Performance
As a globally recognized ophthalmic material, Pharnorcia® VMA (CAS#:3195-78-6) delivers exceptional performance in international markets.
Preferred by International Brands
Pharnorcia® VMA (CAS#:3195-78-6) has become a core material for world-class brands such as Johnson & Johnson, Bausch + Lomb, and Alcon, highlighting its leadership in the industry.
Comprehensive Global Supply Network
With a mature supply chain management system, Pharnorcia® successfully exports VMA to North America, Europe, Asia, and beyond, ensuring efficient service and support for customers worldwide.
Driving Industry Innovation
Thanks to its exceptional properties, VMA enables ophthalmic companies to develop more competitive, high-end products, enhancing the end-user experience while driving industry advancements.
Quality Assurance and Support
Pharnorcia® adheres to strict quality management practices, ensuring every batch of VMA (CAS#:3195-78-6) meets international industry standards. In addition, the company provides comprehensive technical support and after-sales services to help customers reduce uncertainties during product development.
Pharnorcia® VMA (CAS#:3195-78-6) has become the trusted choice for global ophthalmic companies due to its internationally certified quality, outstanding performance, and extensive global applications. Whether developing contact lenses or other optical products, VMA provides robust technical support and quality assurance to meet your needs.
Contact Information:
Email: gm@osigen.com.cn
Phone: +86-13911254076
Website: www.pharnorcia.com
 【Scan code】
【Scan code】

WeChat identification QR code